Title: Hawaii Officials Discuss Ways State Can Petition DEA For Exception To Federal Marijuana Schedule I Classification
Sourced From: weedpress.wordpress.com/2021/03/25/hawaii-officials-discuss-ways-state-can-petition-dea-for-exception-to-federal-marijuana-schedule-i-classification/
Published Date: Fri, 26 Mar 2021 05:30:39 +0000
The roughly 2,000 Facebook followers and email subscribers here at WeedPress should be intrigued. On Tuesday this week, Hawaii’s Health, Human Services, and Homelessness committee discussed Hawaii House Concurrent Resolution 132. HCR 132 directs Hawaii officials to begin the process of “requesting the Department of Health to submit a request to the Drug Enforcement Administration for an exception to regulations and a petition to initiate proceedings for federal rulemaking to clarify that the state-authorized use of medical cannabis does not violate the federal controlled substances act.”
Below is recent discussion by Hawaii officials, as well as the full text of the proposal. Discussion is two minutes; the text is roughly one single-spaced page.
To view original video by clicking here: Hawaii House of Representatives YouTube channel.
Special thanks to Kurt Hanna of the Minnesota chapter of Republicans Against Marijuana Prohibition (RAMP) for the find. Follow RAMP_MN on Twitter. To read more on Minnesota’s effort

Similar legislation is being advanced and discussed in Minnesota. Iowa officials have also agreed to apply to DEA for a federal exemption as well. Click here to follow WeedPress on Facebook for more upcoming articles as we continue our 12 year effort to end this unnecessary conflict between state and federal law — and bring law and order to otherwise lawful state medical marijuana industries.
Read more at WeedPress: Minnesota Bill Requireing Minnesota’s Medical Marijuana Progrm Be Exempted From Federal Law ADVANCES
Read more at Marijuana Moment: Iowa Officials To Seek Federal Marijuana Exemption From DEA
Other medical cannabis states are on board with following this already provided for legal remedy, most notably those of Iowa and Minnesota, who have been leading the effort by the states to properly exempt state medical marijuana industry from federal laws.
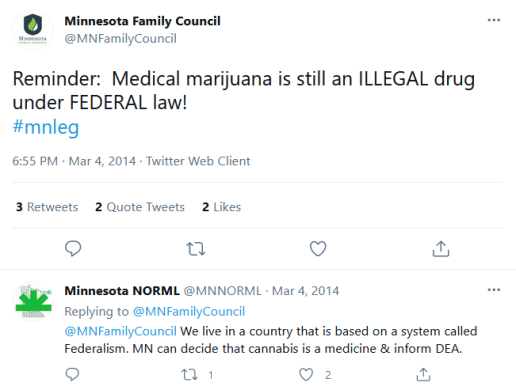
Back in 2014, the Minnesota Family Council (@MNFamilyCouncil on twitter), claimed that state marijuana laws are seemingly in violation of federal law. See the Family Council’s 2014 tweet on the right. Glad to report the Council should be satisfied that there is a solution advancing in cold Minnesota to the Minnesota Family Council’s wisely perceived problem.
Our current system of government already allows a process and solution to solve this the conflict between state and federal marijuana laws, and these three states, Hawaii, Iowa, and Minnesota, are in the lead to use this solution, 26 years after the first medical marijuana law was passed to allow for compassionate marijuana medicines to be provided to patients who otherwise could not find adequate relief for their medical conditions.
As the Hawaii House Health, Human Services, & Homelessness Committee discussed this past Tuesday March 23, the solution to the Minnesota Family Council’s problem with medical marijuana is found in Title 21 Code of Federal Regulations section 1307.03, which allows the Administrator of the Drug Enforcement Administration to grant exceptions to certain federal regulations.
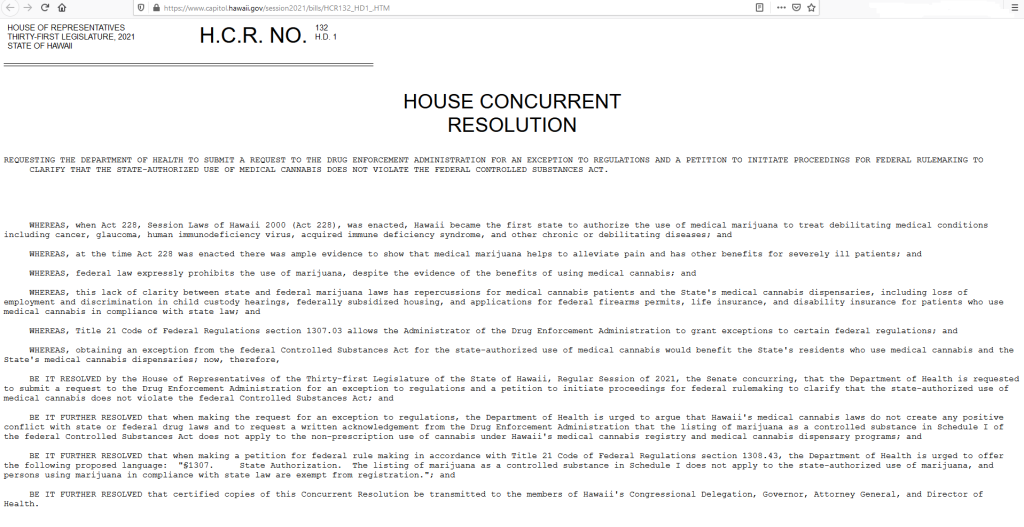
Hawaii’s language also states “BE IT FURTHER RESOLVED that when making a petition for federal rule making in accordance with Title 21 Code of Federal Regulations section 1308.43, the Department of Health is urged to offer the following proposed language: “§1307. State Authorization. The listing of marijuana as a controlled substance in Schedule I does not apply to the state-authorized use of marijuana, and persons using marijuana in compliance with state law are exempt from registration.”” Read the full text of HCR 132 below:
| HOUSE OF REPRESENTATIVES | H.C.R. NO. | 132 |
| THIRTY-FIRST LEGISLATURE, 2021 | H.D. 1 | |
| STATE OF HAWAII | ||
HOUSE CONCURRENT
RESOLUTION
REQUESTING THE DEPARTMENT OF HEALTH TO SUBMIT A REQUEST TO THE DRUG ENFORCEMENT ADMINISTRATION FOR AN EXCEPTION TO REGULATIONS AND A PETITION TO INITIATE PROCEEDINGS FOR FEDERAL RULEMAKING TO CLARIFY THAT THE STATE-AUTHORIZED USE OF MEDICAL CANNABIS DOES NOT VIOLATE THE FEDERAL CONTROLLED SUBSTANCES ACT.
WHEREAS, when Act 228, Session Laws of Hawaii 2000 (Act 228), was enacted, Hawaii became the first state to authorize the use of medical marijuana to treat debilitating medical conditions including cancer, glaucoma, human immunodeficiency virus, acquired immune deficiency syndrome, and other chronic or debilitating diseases; and
WHEREAS, at the time Act 228 was enacted there was ample evidence to show that medical marijuana helps to alleviate pain and has other benefits for severely ill patients; and
WHEREAS, federal law expressly prohibits the use of marijuana, despite the evidence of the benefits of using medical cannabis; and
WHEREAS, this lack of clarity between state and federal marijuana laws has repercussions for medical cannabis patients and the State’s medical cannabis dispensaries, including loss of employment and discrimination in child custody hearings, federally subsidized housing, and applications for federal firearms permits, life insurance, and disability insurance for patients who use medical cannabis in compliance with state law; and
WHEREAS, Title 21 Code of Federal Regulations section 1307.03 allows the Administrator of the Drug Enforcement Administration to grant exceptions to certain federal regulations; and
WHEREAS, obtaining an exception from the federal Controlled Substances Act for the state-authorized use of medical cannabis would benefit the State’s residents who use medical cannabis and the State’s medical cannabis dispensaries; now, therefore,
BE IT RESOLVED by the House of Representatives of the Thirty-first Legislature of the State of Hawaii, Regular Session of 2021, the Senate concurring, that the Department of Health is requested to submit a request to the Drug Enforcement Administration for an exception to regulations and a petition to initiate proceedings for federal rulemaking to clarify that the state-authorized use of medical cannabis does not violate the federal Controlled Substances Act; and
BE IT FURTHER RESOLVED that when making the request for an exception to regulations, the Department of Health is urged to argue that Hawaii’s medical cannabis laws do not create any positive conflict with state or federal drug laws and to request a written acknowledgement from the Drug Enforcement Administration that the listing of marijuana as a controlled substance in Schedule I of the federal Controlled Substances Act does not apply to the non-prescription use of cannabis under Hawaii’s medical cannabis registry and medical cannabis dispensary programs; and
BE IT FURTHER RESOLVED that when making a petition for federal rule making in accordance with Title 21 Code of Federal Regulations section 1308.43, the Department of Health is urged to offer the following proposed language: “§1307. State Authorization. The listing of marijuana as a controlled substance in Schedule I does not apply to the state-authorized use of marijuana, and persons using marijuana in compliance with state law are exempt from registration.”; and
BE IT FURTHER RESOLVED that certified copies of this Concurrent Resolution be transmitted to the members of Hawaii’s Congressional Delegation, Governor, Attorney General, and Director of Health.




 customers.
customers. 3/22/2021
3/22/2021
